Summary of the research group’s field of study
The work of the research group can be divided in four topics, that are:
Photoelectrochemical processes at the nanoscale, new methodologies.
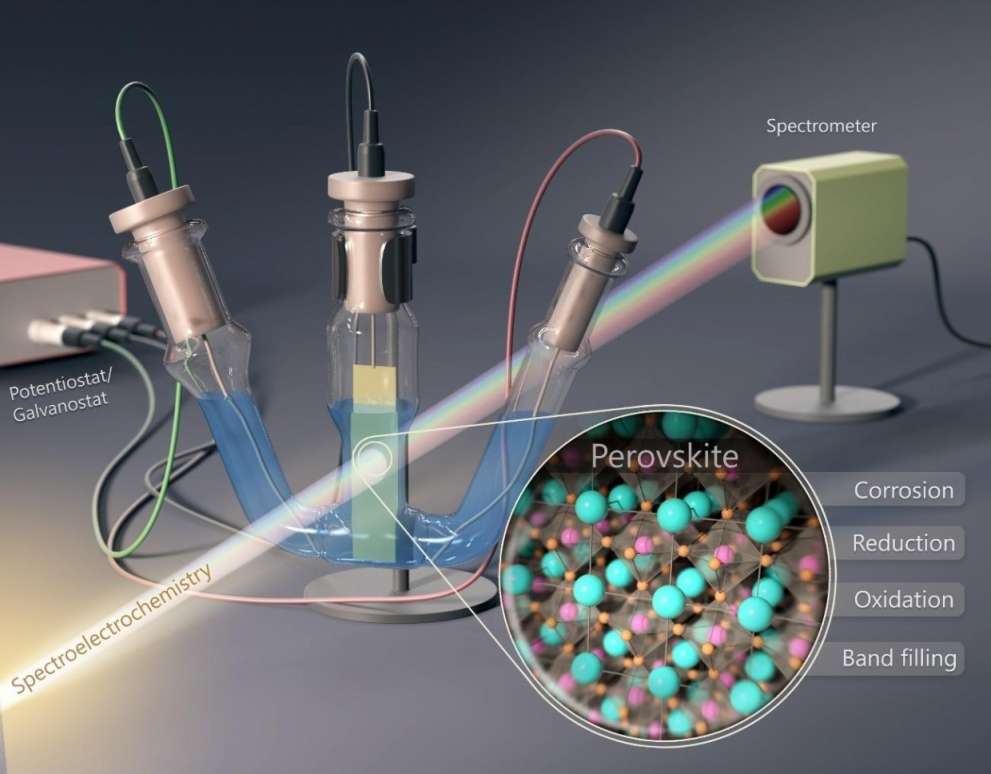
We develop and implement in situ (photo)electrochemical methods, where the electrochemical information is complemented with either spectroscopic or microscopic data. By having at least two independent pieces of information on the same phenomenon, light induced processes in semiconductor electrodes can be better understood.
Photoelectrochemical solar fuel generation.
We employ hybrid materials in photoelectrochemical cells to generate solar fuels. The aim is to understand the function of each component, and maximize the synergy of hybrid formation. Our focus is on the reduction of CO2, thus we develop photocathode for this purpose, as well as photoanodes for the conjugate reaction (water oxidation).
Continuous-flow photoelectrochemical cells.
We develop electrolyzer cells to investigate photoelectrochemical processes. We study the effect of different parameters (e.g., the cell structure, magnitude of irradiance, temperature) on the rate, stability, and selectivity of electrochemical reduction/oxidation reaction (e.g., glycerol oxidation).
Perovskite (photo)electrochemistry.
We perform electrochemical and photoelectrochemical experiments on optically active lead halide perovskite materials. The goal is to better understand their optoelectronic properties, identify degradations mechanisms, and ultimately employ them as photoelectrodes in solar fuels generation.
The most important scientific results of the research group
Detailed study of semiconductors is performed for deep understanding of the optoelectronic features which is a key factor in designing efficient and stable photoelectrodes. In situ spectroelectrochemical methods were employed to analyze the effect of trap states on the optical and electronic properties of photoelectrodes and to assess their stability against (photo)electrochemical corrosion (Cu2O, CuI, NiO, TiO2).
Harvesting the possible synergies of the hybrid formation between an oxide semiconductor with a highly conductive nanocarbon framework (such as graphene or carbon nanotubes) efficient photoelectrodes have assembled for solar fuel generation. We have designed and prepared several hybrid structures of electrodes to study the photoelectrochemical activity, and charge carrier dynamics (FeNiOOH/Fe2O3/graphene, Cu2O/graphene).
We have prepared two-dimensional nanostructures (MoSe2, WSe2) using exfoliation techniques with catalytic centres, to understand their electronic and structural properties unravelling the role of defects for photoelectrochemical performance. We determined by Pt nanoparticles deposition on WSe2 nanoflakes that, the passivation of trap states, activation of catalytic active centres, or both are required to improve the photoelectrochemical activity.
We have applied electrochemical, spectroelectrochemical, and photoelectrochemical methods for analyzing the optoelectronic features of lead halide perovskites and for assembling them into hybrid architectures (e.g., solar cells). At the same time, the instability of these materials limits the pool of solvents and electrolytes that can be employed in such experiments. The focus of our studies is to establish a stability window for electrochemical tests for all-inorganic CsPbBr3 and hybrid organic–inorganic MAPbI3 perovskites. In addition, we aimed to understand the reduction and oxidation events that occur and to assess the damage done during these processes at extreme electrochemical conditions.
Results of the last 1 year
The research group studies the light induced processes in semiconductor materials. We develop and implement in situ methods to investigate the photoelectrochemical activity, and charge carrier dynamics of semiconductors. We prepare novel hybrid materials and employ these in our self-constructed photoelectrochemical cells to generate solar fuels.
ACS Energy Lett. 2022, 7, 1, 417–424.
J. Phys. Chem. C 2021, 125, 14, 7701–7710.
Adv. Funct. Mater. 2020, 30, 2002124.
The 5 most important publications of the research
- H. W. Jeong, T. S. Zsigmond, G. F. Samu, C. Janáky, Sacrificial Agent Gone Rogue: Electron-Acceptor-Induced Degradation of CsPbBr3 Photocathodes, ACS energy Lett., 2021, 7, 417-424.
https://doi.org/10.1021/acsenergylett.1c02130 - A. Kormányos, E. Kecsenovity, A. Honarfar, T. Pullerits, C. Janáky, Hybrid FeNiOOH/α‐Fe2O3/Graphene Photoelectrodes with Advanced Water Oxidation Performance, Adv. Funct. Mater., 2020, 30, 2002124. https://doi.org/10.1002/adfm.202002124
- Á. Balog, G. F. Samu, P. V. Kamat, C. Janáky, Optoelectronic Properties of CuI Photoelectrodes, J. Phys. Chem. Lett., 2019, 10, 259-264. https://doi.org/10.1021/acs.jpclett.8b03242
- G. F. Samu, Á. Balog, F. De Angelis, D. Meggiolaro, P. V. Kamat, C. Janáky, Electrochemical hole injection selectively expels iodide from mixed halide perovskite films, JACS, 2019, 141, 10812-10820. https://doi.org/10.1021/jacs.9b04568
- E. Kecsenovity, B. Endrődi, P. S. Tóth, Y. Zou, R. A. W. Dryfe, K. Rajeshwar, C. Janáky, Enhanced photoelectrochemical performance of cuprous oxide/graphene nanohybrids, JACS, 2017, 139, 6682-6692.
https://doi.org/10.1021/jacs.7b01820
Head of the research group: Dr. Csaba Janáky

Members of the Research Group:
Dr. Ádám Balog
Dorottya Hursán
Dr. Hye Won Jeong
John Mark Christian Dela Cruz
Dr. Gergely Ferenc Samu
Dr. Péter Sándor Tóth
Xiangtian Chen
The most important tools
- Autolab PGSTAT204, 302, 302N potentiostat/galvanostat workstations, Rotating disk electrode, IMPS, EQCM setups, 10 A current booster
- Shimadzu GC-2030 gas chromatograph equipped with high temperature automatized sampler
- SRS UGA200 atmospheric sampling mass spectrometer
- Nikon Eclipse LV100ND optical microscope, SMZ745T stereomicroscope
- Solar simulator: Oriel (Newport), LCS-100
- IPCE setup: Newport, QEPVSI-b
- Spectrophotometers: HP8452A, Avantes Spec2048
- ALD equipment (Beneq TFS 200 ALD) installed in a clean room
- Kelvin probe (KP Technology APS04)
- custom-designed micro-scale photoelectrochemical setup, allowing us to study the (photo)electrochemical behavior under illumination with controlled wavelength and intensity.
The most important figures connected to the research fields

Industrial partners
- ELI-ALPS https://www.eli-alps.hu/
Contact the research group leader on the following e-mail address:
janaky@chem.u-szeged.hu
